Automated Regulatory Authoring for Life-Critical Medical Documentation
Regulatory Intelligence
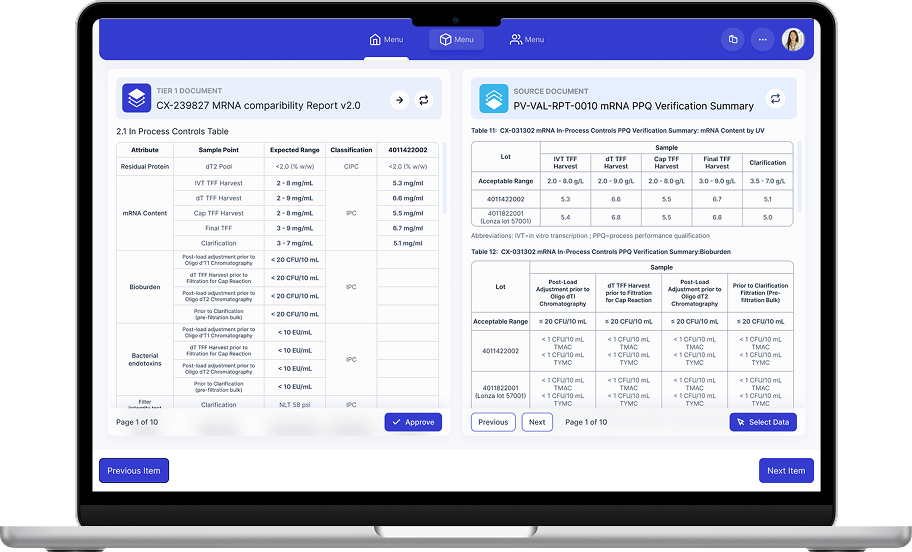
Designed an automated regulatory authoring platform for high-stakes medical documentation, including mRNA vaccine specifications and validation summaries. The system automates writing and data transfer across complex regulatory documents without sacrificing trust, traceability, or human approval.
Medical regulatory documentation is:
- -Extremely dense and interconnected
- -Manually copied across multiple source documents
- -Zero tolerance for errors or ambiguity
- -Existing tools treat these documents like text
They are structured systems with scientific constraints.
Design a platform that:
Automation could assist, but never replace, human judgment.
Side-by-Side Authoring Workspace
Target document and source document shown simultaneously with synchronized navigation for fast verification and visual comparison of acceptable ranges vs actual values.
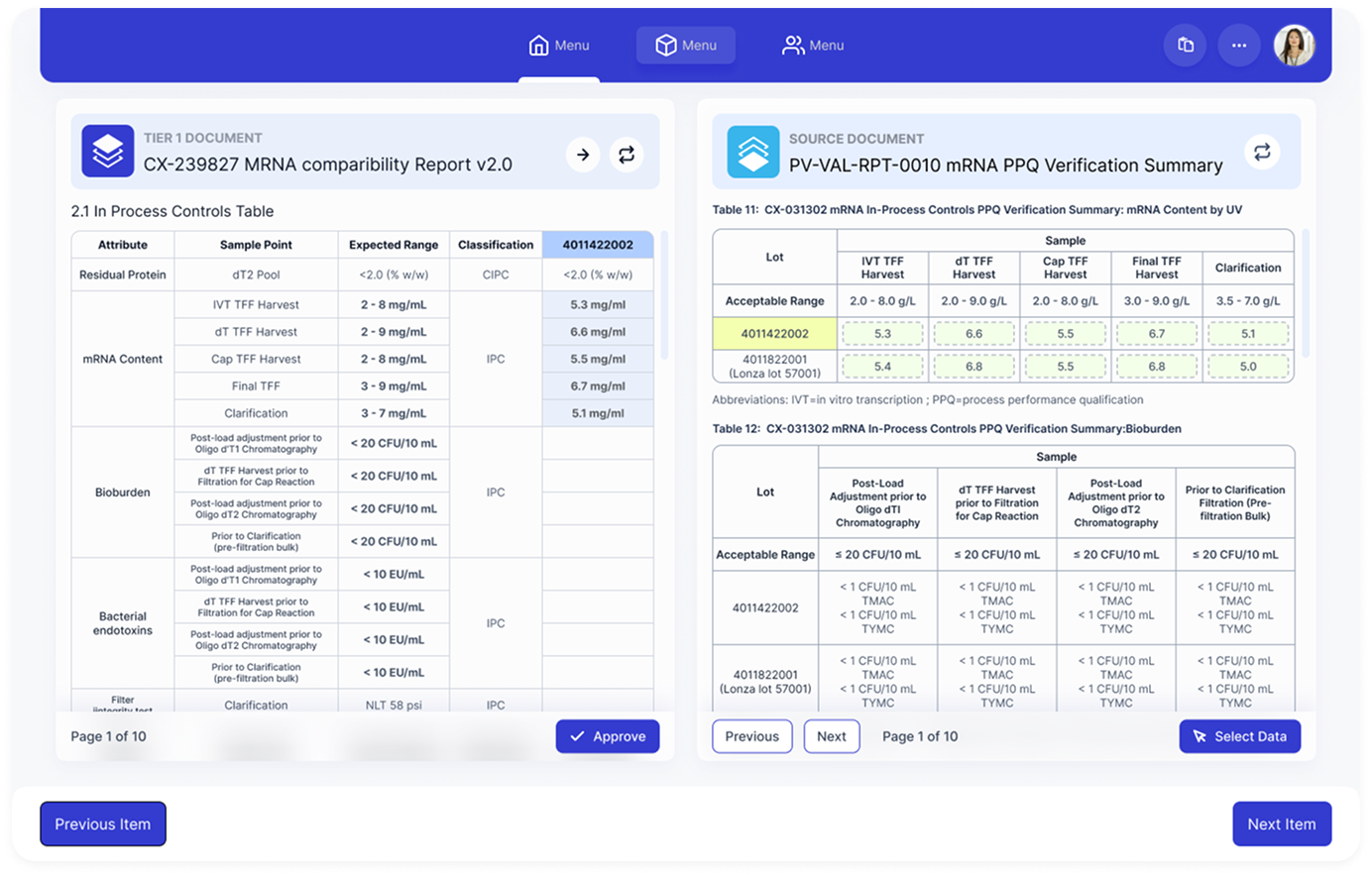
- - 2.1 In Process Controls Table
- - Residual Protein, mRNA Content, Bioburden
- - Bacterial endotoxins, Filter Integrity
- - Table 11: mRNA In-Process Controls
- - Table 12: Bioburden Summary
- - Sample data across multiple lots
Structured Data Extraction
Values extracted as data, not text. Each value retains source document, table, and page reference enabling validation, reuse, and reviewer confidence.
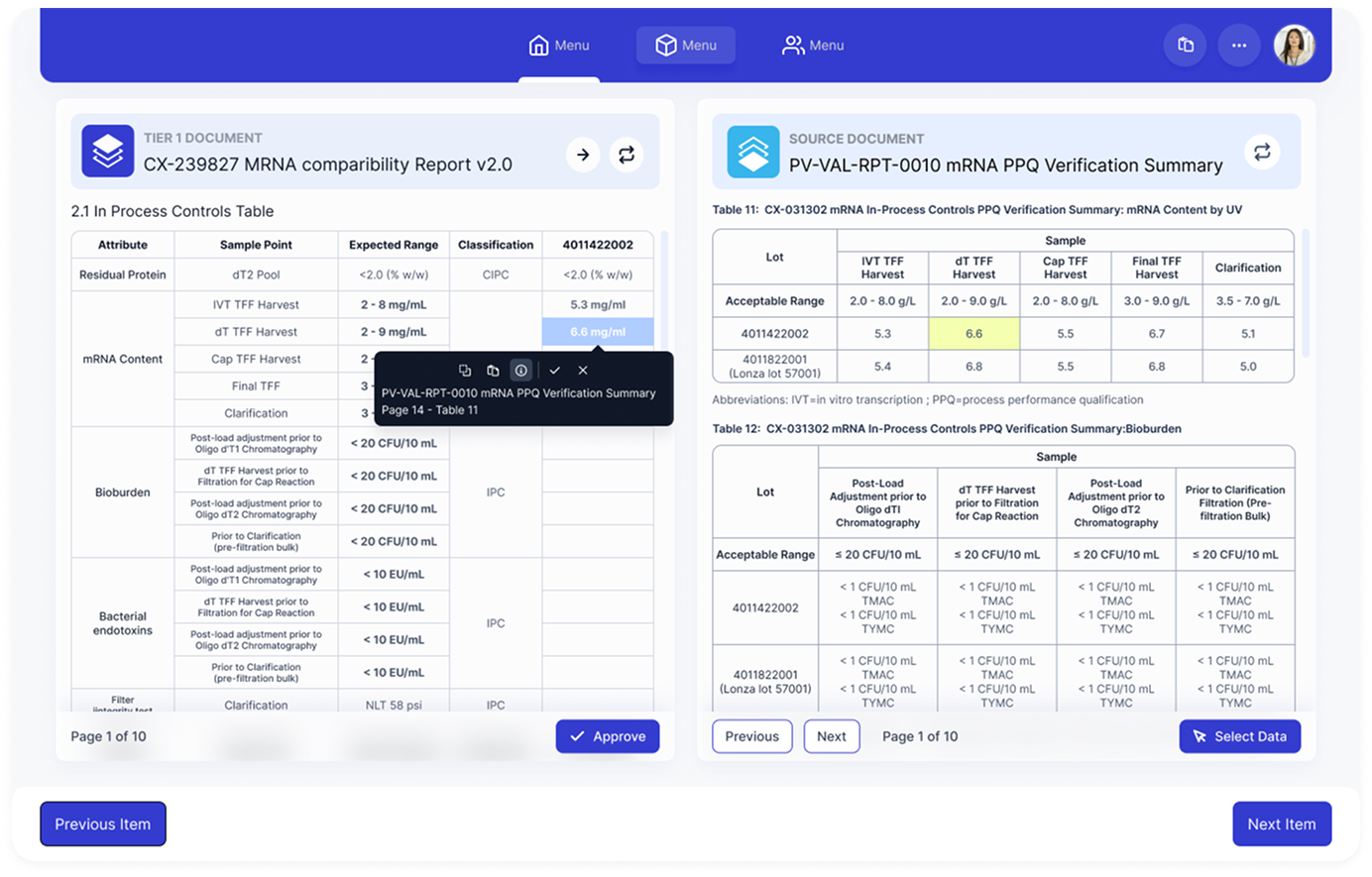
Every data point maintains full provenance, allowing reviewers to verify source material with a single click.
Approval-First UX
Every data point requires explicit human approval. Clear approved, pending, and rejected states with no silent automation.
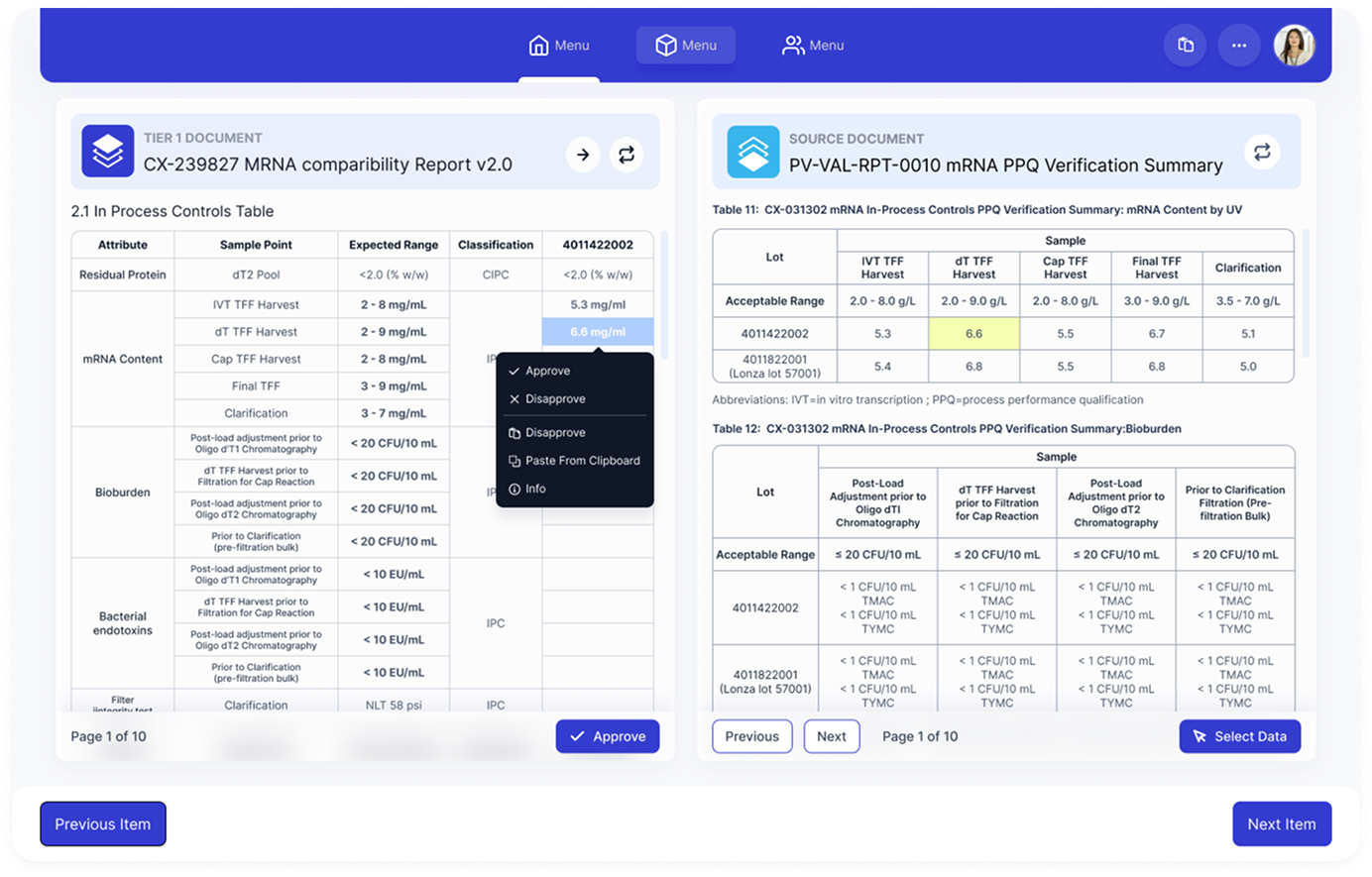
Contextual Actions
Approve, disapprove, inspect source, or replace at the cell level. Mirrors how scientists already review data.
AutoWriter mRNA Documentation System
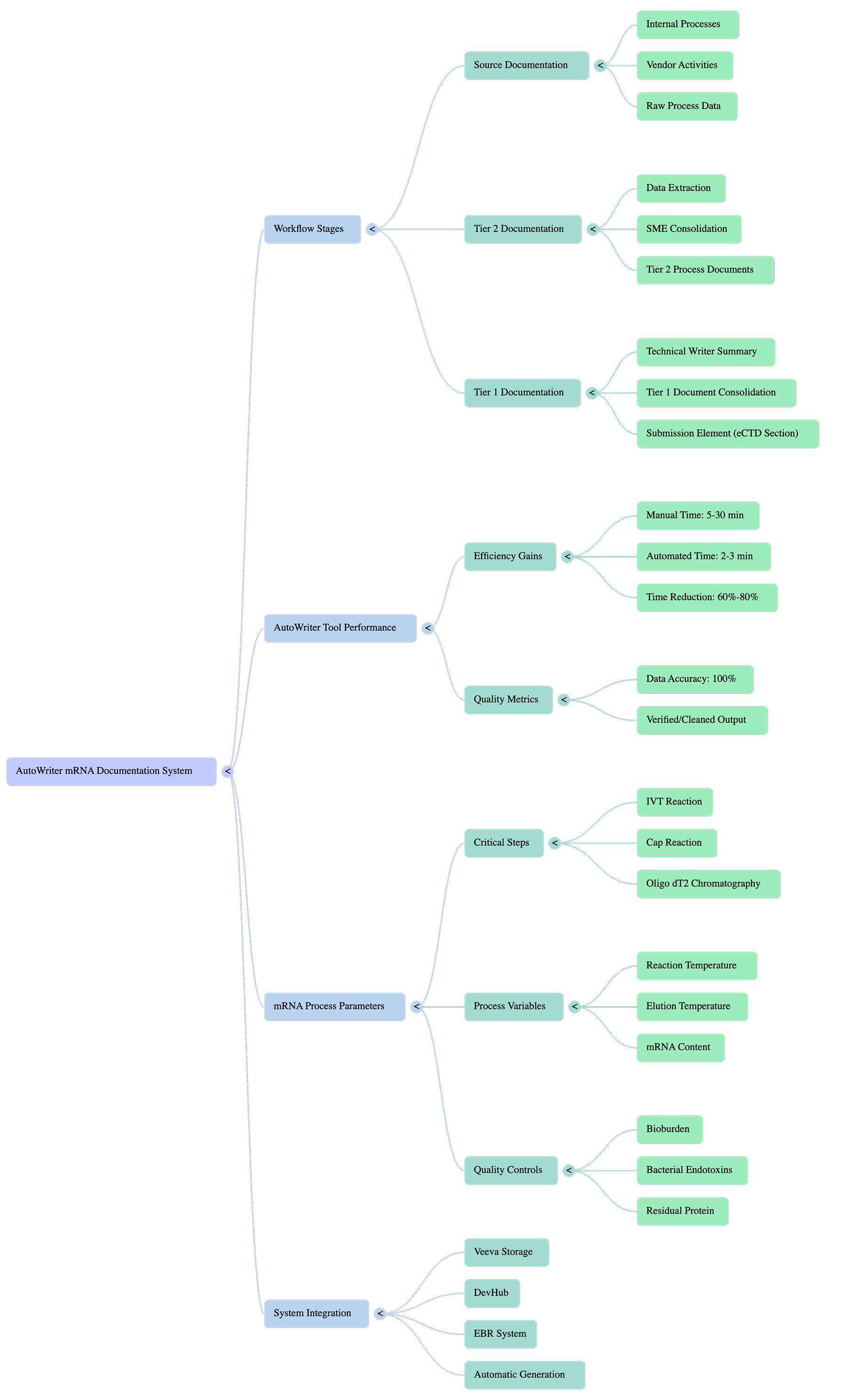
- - Source Documentation
- - Tier 2 Documentation
- - Tier 1 Documentation
- - Data Extraction & SME Consolidation
- - Manual Time: 5-58 min
- - Automated Time: 2-3 min
- - Time Reduction: 60%-80%
- - Data Accuracy: 100%
- - IVT Reaction
- - Cap Reaction
- - Oligo dT2 Chromatography
- - Quality Controls
- - Nexus Storage
- - OpenBio
- - EBR System
- - Automatic Generation
- - Clean, professional interface for regulatory workflows
- - Light theme with blue/purple accents for trust
- - Dense data tables optimized for scientific review
- - Clear visual hierarchy between target and source
- - Green checkmarks for approved, yellow for pending
- - Contextual tooltips preserve focus